Improving blood safety is a current topic: 315 participants from 55 countries at BloodTrain seminar series on haemovigilance
The BloodTrain team has been systematically strengthening haemovigilance within its partner countries since 2019. The BloodTrain seminar series focused on establishing an effective monitoring system for blood safety, sharing experiences of other countries and the challenges that need to be overcome. It occurred online from the 5 to 7 September 2023.

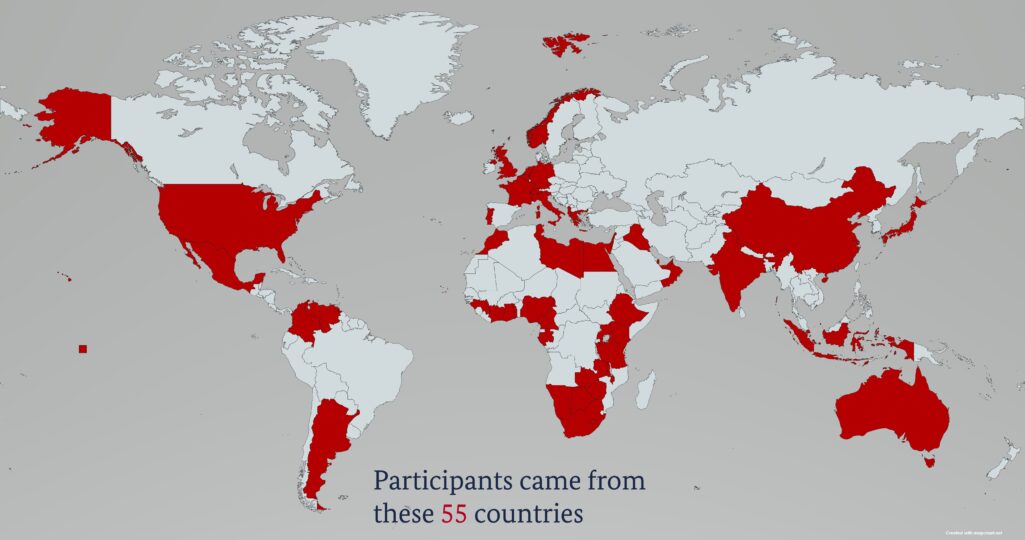
Haemovigilance comprises a complex system for monitoring the entire blood transfusion chain from blood collection to transfusion and the follow-up of transfused patients. It serves to ensure the safety and improved use of blood and blood products. By working on the development of haemovigilance systems in Africa, BloodTrain has gained deeper insight into different national contexts in low- and middle-income countries. On this basis, the team designed a customised workshop that focused on important steps, critical points and pitfalls. Nine international haemovigilance experts shared their knowledge and experiences with the participants in 16 practical presentations and were available afterwards for in-depth discussion sessions. The BloodTrain project lead, Dr Jens Reinhardt, summarizes: “With the high-profile online workshop, we were able to further broaden the knowledge of haemovigilance and make it accessible to other interested countries. The fact that the workshop was very well attended with 315 participants from 55 countries once again emphasizes the relevance and topicality of our activities in the field of blood safety.” BloodTrain subsequently made available the recordings of the presentations to its partner countries. All participants received the presentations as PDF files.
Three content blocks: Basics, establishing and performing haemovigilance
The seminar series consisted of three blocks. The first part was an overview of the aims of a haemovigilance system (HV), its organization and details of the basic constituents of a HV system. These include, for example, reporting channels for adverse events, causality assessments and the tracing of events. Dr Philipp Berg from the Paul-Ehrlich-Institut, the higher federal authority in Germany that collects and evaluates reports of adverse events in the blood transfusion chain, pointed out that a transparent official hemovigilance reporting system increases the willingness of stakeholders to report events. Hemovigilance encompasses different players and it is important to disclose in the reports what the authority does with the data it receives. For example, figures on serious transfusion incidents or new infectious agents when regularly analyzed and published, create the basis for improvements in the system.
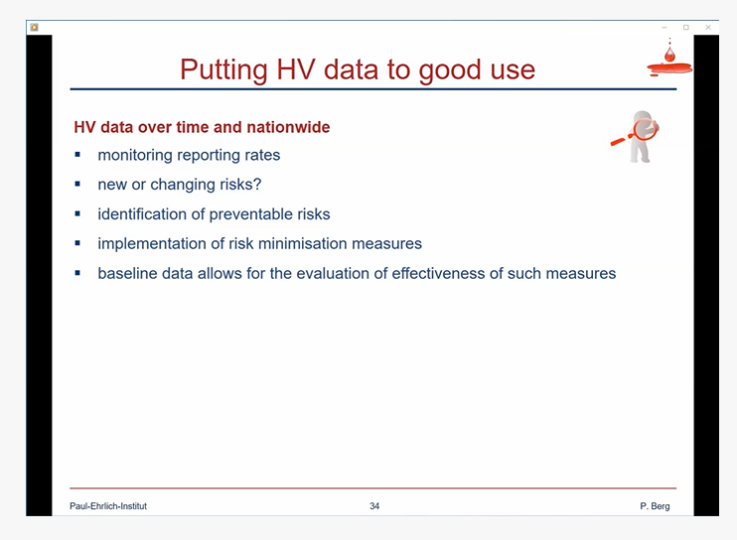
The goal of haemovigilance is continuous improvement of the blood transfusion chain
Dr Yuyun Maryuningsih, from the Blood and Other Products of Human Origin Section at the World Health Organisation (WHO) headquarters in Geneva, emphasized precisely the goal of a haemovigilance system, namely the continuous improvement of the blood transfusion chain. Her presentation marked the start of the second session, in which participants were taken through concrete approaches to setting up a haemovigilance system in their countries. Dr Maryuningsih explained the various guidelines developed by WHO for guidance and a step-by-step approach to implementation. In three other presentations, speakers from India, Namibia and Zambia elucidated their approach to setting up a haemovigilance system and shared their experiences. For example, in India, it took several years to set up the haemovigilance system which is based on phased implementation model. Or the importance of involving and training healthcare workers when establishing haemovigilance systems was emphasized. This could significantly improve the reporting of events.
Risk Minimization through Teamwork
In the third session, speakers from the three groups that ideally work together in a haemovigilance system reported on their specific work to improve blood safety: from transfusing facilities, to the blood establishment that collects, tests and usually processes the donated blood into blood components, and the regulatory authority. Dr Michael Schmidt, Professor of Transfusion Medicine at the University Hospital of the Johann Wolfgang Goethe- University in Frankfurt and the German Red Cross, focused on the safety of both donors and recipients. In his presentation, he illustrated how cases of bacterial or viral transmission through transfusion have led to significant changes in requirements for storage times or testing procedures through traceability and causal research. He also emphasized the need for optimal cooperation between hospitals, blood donation services and authorities in order to continuously minimize risks. A full list of speakers and a link to the agenda can be found below.
Speakers:
Dr. Philipp Berg, Paul-Ehrlich-Institut
Lesley Bust, Africa Society for Blood Transfusion
Dr. N. Choudhury, Asian Association of Transfusion Medicine
Dr. Yuyun Maryuningsih, World Health Organization
Dr. Faten Moftah, Africa Society for Blood Transfusion
Dr. Susanne Müller, Paul-Ehrlich-Institut
Dr. Joseph Mulenga, Zambia National Blood Transfusion Service
Dr. Shruthi Narayan, Serious Hazards of Transfusion
Washington Samukange, Enabel-Belgian Development Agency
Dr. Michael Schmidt, Deutsches Rotes Kreuz & Klinikum der Johann Wolfgang Goethe-Universität Frankfurt am Main
Dr. Mary Townsend, International Haemovigilance Network / International Society for Blood Transfusion,
Dr. Carla van Zyl, Namibian Blood Transfusion Service
Selection of further links:
English version of the PEI Haemovigilance report from 2016/2017:
Standardized donor questionnaire of PEI
ISBT Haemovigilance Tools and resources