NiCaDe-Hep/Rota: Activation of Sentinel Sites
The planned start of hepatitis and rotavirus sample collection in late 2019/early 2020 had to be postponed due to the COVID-19 pandemic-related delays in the project beginning in 2020. Since November 2020, the sentinel sites have been activated to collect samples as part of the intensified molecular surveillance of hepatitis and rotavirus infections in Nigeria.

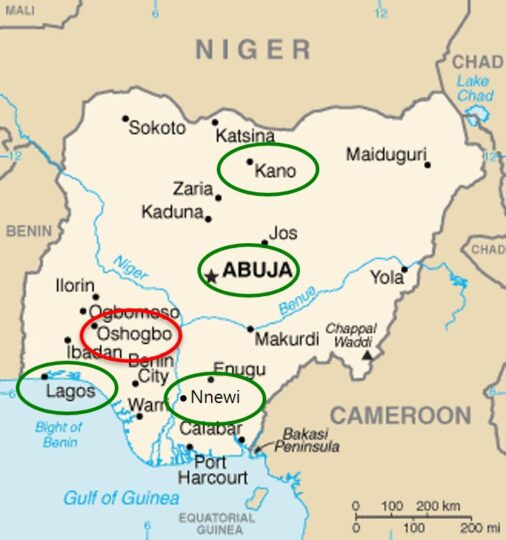
The COVID-19 pandemic poses an unprecedented challenge to healthcare systems worldwide. A cornerstone of successful containment is rapid and reliable diagnostics. The intensified molecular surveillance of hepatitis and rotavirus infections with a network of sentinel sites distributed across Nigeria can serve as the basis for a rapidly available, functioning surveillance system also in the event of an outbreak of other pathogens.
The close involvement of team members from the NCDC, the sentinel sites and the RKI in managing the pandemic as well as measures like contact and travel restrictions severely impacted planned project activities in the first six months of 2020. Despite the ongoing pandemic, key project milestones have been achieved in the second half of 2020.
In fall 2020, materials for diagnosing hepatitis and rotavirus infections as well as consent and data-capturing forms have been distributed to the sentinel sites. Subsequently, a team from the NCDC travelled to four of the five sentinel sites between 19 November and 9 December 2020. During interactive workshops methodological and diagnostic aspects of the project have been refreshed and challenges of each sentinel site regarding collection, storage and transport of samples to the NCDC were discussed and solutions were developed.
By the end of 2020, the first samples of individuals with suspected hepatitis or rotavirus infection were available. Following initial antigen/antibody rapid tests at the sentinel sites, the samples are subsequently undergoing further molecular biologic testing and characterization at the NCDC’s National Reference Laboratory.
Date: January 2021