West Africa expands its sequencing capacity
In 2023, eight scientific staff from Guinea and Nigeria have been trained on a new sequencing methodology, reinforcing their in-country genomic surveillance capacities to support public health systems.

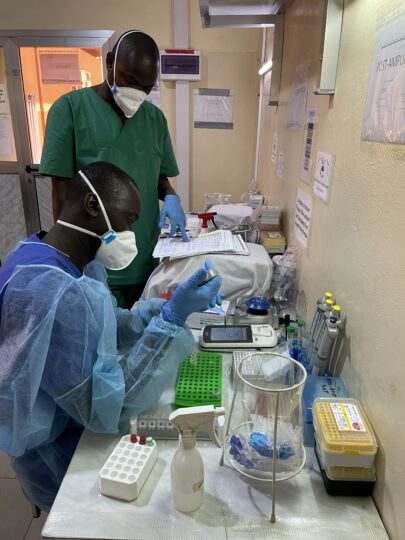
As part of this new programme phase of GHPP (2023-2025), our project CELESTA focuses on expanding the genomic surveillance capacity for RNA (ribonucleic acid) viruses of our cooperation partners. They are: the Laboratoire de Centre de Recherche en Virologie – Laboratoire des Fièvres Hemorragiques Virales de Guinée (CRV-LFHVG) in Conakry, Guinea, the Laboratoire des Fièvres Hémorragiques Virales de Gueckédou (LFHV-GKD) in Gueckédou, Guinea, and the Irrua Specialist Teaching Hospital (ISTH) in Irrua, Nigeria. The BNITM team developed a scalable metagenomic sequencing training curriculum to address both the hands-on bench work and the bioinformatics analysis of sequencing data.
In February 2023, a team from BNITM travelled to CRV-LFHVG for a two-weeks phase-I training using nanopore sequencing (MinION, Oxford Nanopore Technologies). A total of four staff from CRV-LFHVG attended the training. Two of them already had prior knowledge about some technique of next generation sequencing, the so-called amplicon-based sequencing. It was implemented in the laboratory to monitor SARS-CoV-2 variant’s circulation in Guinea in 2021.
In June 2023, four ISTH laboratory scientists and four from CRV-LFHVG participated in a 2-weeks training on metagenomics sequencing and phylogeny, which was organised by the BNITM and hosted in Conakry. As such, they built on the previously established Nigeria-Guinea cooperation in the field of SARS-CoV-2 sequencing. To further strengthen sequencing capacity development in Guinea, the BNITM team facilitated another training in Gueckédou in November 2023. A total of six LFHV-GKD staff joined an introductory course on sequencing technologies and bioinformatics.
The main goal of these training sessions was to prime local resources with a new method of next-generation sequencing, which is metagenomic sequencing. Besides, the aim was to familiarise them with data analysis tools using command lines to ultimately implement this new methodology in the respective laboratories. A total of ten new standard operating procedures (SOPs) were implemented for this process, and eight staff from ISTH and CRV-LFHVG were trained accordingly. Emphasis was laid on the following subject matters: processes to strictly follow to minimise risks of contaminations, controls to be used to monitor sequencing efficiency and ensure delivery of data at quality, as well as troubleshooting management. All eight trainees successfully participated in this phase-I programme. Consecutive training phases will follow in 2024.
Both CRV-LFHVG and ISTH now have a basic capacity to perform metagenomic next generation sequencing and related analysis (i.e., consensus sequence) of RNA viruses in-country. This is a key achievement to support the Guinean and Nigerian public health surveillance systems.