From E(vidence) to R(ecommendation) – a Guidance
The EURO NITAGs project team from the Robert Koch Institute and the WHO Regional Office for Europe has published a “Guidance on an adapted Evidence to Recommendation Process” for recently established National Immunization Technical Advisory Groups of the WHO European Region that are faced with limited resources.
National Immunization Technical Advisory Groups (NITAGs) are advisory bodies that develop recommendations for policy maker to support them in taking country-owned, evidence-based, and sustainable decisions on immunization policies. Long-standing NITAGs such as the German NITAG “STIKO”, the UK “Joint Committee on Vaccination and Immunisation” (JCVI) or the US “Advisory Committee on Immunization Practices” (ACIP) develop their recommendations using the gold standard of recommendation making process, the so-called Evidence to Recommendation (EtR) process.
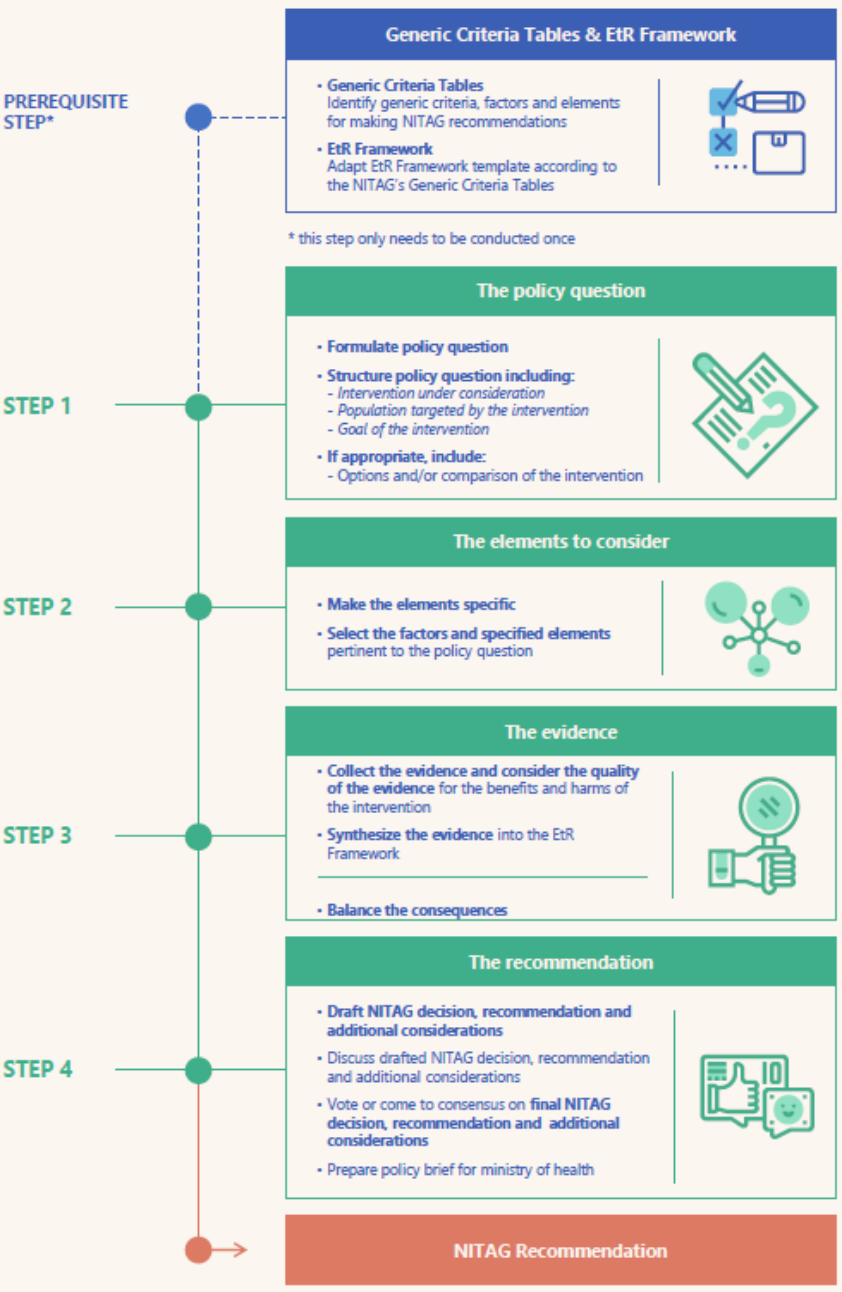
For recently established NITAGs in the WHO European Region, that often face limited human and financial resources, applying the “gold standard” is very challenging and many of these NITAGs do neither apply a systematic nor an evidence-based approach but develop their recommendations based on expert opinions. To address these challenges and encourage the use of a systematic process the EURO NITAGs project team from the Robert Koch Institute and the WHO Regional Office for Europe developed a “Guidance on an adapted Evidence to Recommendation Process for National Immunization Technical Advisory Groups” that is adapted to fit the level of maturity of recently established NITAGs and acknowledges their constraints in capacities and resources.

The adapted EtR Process is based on the “gold standard” used by long-functioning NITAGs but does intentionally not include the conduct of a systematic literature review. Such reviews are done to systematically summarize the evidence for the benefits and harms of an intervention and are the most extensive and complex part of the entire recommendation process. Because the preparation of systematic reviews requires sufficient human resources and expertise, NITAGs with limited time and human resources are encouraged to use existing systematic literature reviews conducted by other NITAGs or institutions, that are summarized and qualitatively assessed in the SYSVAC database (see also GHPP-SYSVAC).
The “Guidance on an adapted Evidence to Recommendation Process for National Immunization Technical Advisory Groups” has now been published in English and Russian on the WHO Europe website and is currently piloted by the NITAGs of Armenia, Republic of Moldova and Uzbekistan in their recommendation-making process. The EURO NITAGs project team supports these NITAGs during the process with regular webinars, in which each step of the EtR process is presented and applied to the NITAGs specific topic under discussion.
Results and lessons learned from the process will be presented by the NITAGs at the Programs Managers Meetings to be held in the region from September to November 2022.
Date: September 2022


